 Musk impart sensuality to perfume it bring volume and diffusitvity and impart warmth and liveliness. I think it's safe to say that there's no perfume without musk. Musk tincture smells animalic, sweet and ammoniacal. But the more one studies its character, the more contrasting, vibrant and oscillating it becomes: repulsive-attractive, chemical-warm, sweaty-balmy, acrid-waxy, earthy-powdery, fatty-chocolate like, pungent-leathery, resinous-spicy, fig-like, dry, nutty, and woody.
Musk impart sensuality to perfume it bring volume and diffusitvity and impart warmth and liveliness. I think it's safe to say that there's no perfume without musk. Musk tincture smells animalic, sweet and ammoniacal. But the more one studies its character, the more contrasting, vibrant and oscillating it becomes: repulsive-attractive, chemical-warm, sweaty-balmy, acrid-waxy, earthy-powdery, fatty-chocolate like, pungent-leathery, resinous-spicy, fig-like, dry, nutty, and woody.Originally the musk was obtained from the male musk deer. The internal pouches found between the hind legs had an intesely smelling secretion. To harvest the secretion the animal was hunted. To obtain 1 kilo of musk grains between 30 and 50 animals had to be sacrificed. Musk tinctures were still used in perfumery till about 1979, when musk deers were protected form extinction by the Conention on International Trade in Endagnered species of wild fauna and flora(CITES)
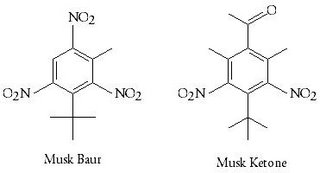
Musky smell refers especially from the smell of the dry-down of the natural musk tincture after the more volatile parts are evaporated and when the more warm, sensual, sweet-powdery tonality comes forward. In 1888 Albert Bauer discovered the nitro musk Musk Bauer. Soon replaced by three other nitro musks that he also discovered: Musk Xylene, Musk Ketone and Musk Ambrette. These musks became really important for the fragrances for the next 50 years.
In 1981 the nitro musks were restricted mainly because of a certain toxicity(neurotoxicity) and their phototoxicity, but besides they also caused ecological concerns due to their poor biodegradability. Ernest Beaux the perfumer that created Chanel no5 used over 10% nitro musks in his formulation mainly Musk Ketone. Musk Ambrette was used by the perfumer Francis Fabron for L'air du Temps. These nitro musks had to be replaced by other musk smelling chemicals and that's not an easy thing to do without changing the smell of these perfumes.

The first nitro free aromatic musk chemical Phantolide was introduced in 1951 by Kurt Fuchs. This was the start of other Polycyclic musks like Celestolide, Fixolide, Tonalide, Galaxolide and many more. Galaxolide was first synthesized in 1965, and already in the late 1960s used in dosages up to 40% in fabric softeners such as Comfort and Soflan and in detergents like Coral at 27%. But high doses were also incorporated in fine fragrances, for instance Tresor by Sophia Grosjsman with its 21,4% of Galaxolide. Galaxolide possesses a clean sweet musky flowery woody odour.
There is another group of chemicals with a musk odour: Macrocyclic musks. Examples of macrocyclic musks are Exaltolide, Habanolide, Velvione and others. Habanolide has a metallic character which is used in Emporio Armani white for her by Alberto Morillas as a component of a white musk accord together with Helvetolide. In Glow by J Lo it's used in an intense white flower accord.
 Nirvanolide a chemical produced by Givaudan has a clean and sweet, powdery and persistent, and slightly animalic odour and is quite close to the restricted Musk Ketone. It's used at 6,7% in the perfume Forever Elizabeth created by David Apel. Another chemical with an odour close to Musk Ketone is Muscenone it possesses a very elegant and diffusive musk odour.
Nirvanolide a chemical produced by Givaudan has a clean and sweet, powdery and persistent, and slightly animalic odour and is quite close to the restricted Musk Ketone. It's used at 6,7% in the perfume Forever Elizabeth created by David Apel. Another chemical with an odour close to Musk Ketone is Muscenone it possesses a very elegant and diffusive musk odour.
Musk odours could also be obtained in the plant kingdom like Angelica root oil that possesses the musk odour Exaltolide(macrocyclic) but also 12-methyl-13-tridecanolide. The discovery of 12-methyl-13-tridecanolide in Angelica root oil was really important because it showed the importance of the effect of methyl substituents on the character of macrocyclic musks. Ambrette seed oil possesses Ambretollide. When thinking about musky smells you don't think about Galbanum because it has a green note with balsamic nuances and isn't musky at all, but the isolated methyl macrolides: 14-pentadecanolide and the 15-hexadecanolide do have a musky odour.
This information I found in the books Chemistry and Technology of flavors and fragrances by David J. Rowe and Perfumery practice and principles by Robert R. Calkin and J. Stephen Jellinek. The picture of the musk pods is from http://www.kogado.co.jp/ the picture of the chemical structures is from http://www.chm.bris.ac.uk/webprojects2003/teo/realmusks.htm the picture of Tresor is from http://www.beautymarkcorp.com/ and the one of Forever Elizabeth is from http://www.fragrancenow.com/










10 comments:
Thank you Jenny! Musks are fascinating and important to me. I appreciate your sharing. gail
You're welcome Gail. What kind of musks do you like to use?
Great article Jenny, I am obsessed with musk and have learned from this article. I guess the substitutes are sufficient for a composition to get it's point across, since it is apparent that synthetics are the only musk substances used today. And it doesn't appear that the industry has suffered from the absence of true musk. Thank you, Frank
I'm glad you like the article Frank, welll synthetics are not the only source there are sources in the plantkingdom as well only not as much as the synthetics.
Thank you for putting that information about musk together into such a wonderful article. Although I have to admit I am really curious as to what Egyptian musk is.
Hi Jennifer, I would like to know that as well, I'm sure it's made of combinations of musks and other notes but which I don't know.
perfectly possible to extract musk without killing the deer. not that they seem to do that any more. idiots. Civet on the other hand...
Hello Jenny,
Really Great Blog!
I accidentally landed on this blog; on this post when searching for Exaltolide. I think I need to communicate more. I had also wet my feet in perfumery a long time back and created some perfumes.
After this post, i read several others. Almost all are fantastic and well organized. However I could not find contact information, therefore I am using the comment
Please get back and we can communicate more. For details, you can check my profile and email from there. I am sure to be delighted to get in touch with you.
hi thanyou for making this blog it has helped me alot with my biotechnology project
http://www.greenpeace.org/raw/content/international/press/reports/phthalates-and-artificial-musk.pdf
Post a Comment